Abstract
Introduction Multiple Myeloma (MM) is an incurable plasma cell malignancy with a median age of 65 years at diagnosis. Although previous studies in "young” MM have been performed, they focus on the clinical characteristics. Within this study, we aim to understand the clinical characteristics and genomic drivers of young MM using the Weill Cornell/New York Presbyterian (WCM) and CoMMpass (MMRF) registry.
Methods Young MM was defined as onset of MM at age <= 45. The WCM MM registry was queried for patient meeting the diagnosis for MM based on International Myeloma Working Group (IMWG). A control population of MM was created by randomly sampling the patients within the registry that were >45 years old. Clinical features and outcomes were recorded for each patient.
The MMRF study is an observational international registry of newly diagnosed MM. A total of 1143 patients had clinical data. Baseline demographic, clinical and laboratory data, along with treatment and genomic (whole exome sequencing, RNA sequencing) was collected, and patients were followed prospectively every 6 months. Patients were divided into <= 45-year group and those above the median age in this cohort (>63 year). The primary end point of our analysis was progression free survival (PFS). Kaplan Meier and univariable Cox proportional hazard model was performed. Multivariable Cox model was created based on the results of univariable model. Land-mark analysis was used to control for time-dependent covariates. All analysis was performed using R software, version 4.1
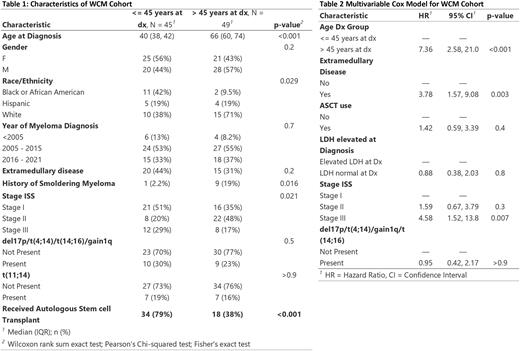
Results The WCM cohort consisted of a total of 179 patients for this study. 61 patients were <= 45 years, and 98 patients belonging to an older control group. The younger-patients group was enriched for individuals of African American descent (40% vs 10%, p = 0.04), stage I ISS (51% vs 34%, p 0.01), more use of autologous stem cell transplant (ASCT), and less frequent prior diagnosis of smoldering myeloma (SM) (2.3% vs 18%, p = 0.017). There was a trend of higher rates of EMD and higher free light chain ratio in the young group, but this was not statistically significant. The median PFS for each group was 7.92 years (95% CI 4.50 - NA) for the younger group and 3.25 years (95% CI 1.92 - 10.67) for the >45-year group. In the univariable model the >45 year had worse PFS (HR 2.4, 95CI 1.2-4.6, p < 0.01). The association of worse PFS in the >45 year group (HR 6.3, 95%CI 2.3-17.2) persisted in the multivariable analysis, in addition to the presence of EMD (HR 3.2, 95% CI 1.4-7.3), and ISS stage (Table 2).
In the MMRF registry, 65 and 548 patients <= 45 years and >63 years respectively were identified. The young group had higher proportion of African American (33% vs 15%, p 0.01), lower ISS (stage I 46% vs 31%, p = 0.04), and less frequency of deletion 13q (35% vs 50%, p 0.03) and gain 1q (22% vs 42%, p < 0.01). t(4;14) was seen more frequently in the young group, but this was not statistically significant (18% vs 9%, p = 0.056). DEA and GSEA showed a total of 1945 (3.5%) and 2164 (3.9%) over and under expressed genes, with the young group being enriched for the CD-1 transcriptional signatures (NES 1.86, adjusted p 0.028). There were no differences in the rates of mutation in the top 23 mutated genes in MM. The median PFS for the young group was 5.0 years (95% CI 2.3 - 5.9) vs 5.4 (95% CI 5.4 - 5.8) years, HR 1.56 (95% CI 1.05-2.3, p = 0.02) on the univariable analysis.
Conclusions Young patients with MM have distinct clinical and demographic characteristics, including higher rates in African Americans, lower ISS stage, and lower rates of deletion 13q and gain 1q and enrichment in the CD-1 MM signature suggesting a potential role genetic and environmental factors in the early presentation at diagnosis. However, young patients had comparable to improved PFS than older patients that could be attributed to both biologic drivers, or factors related to patient age, such as better performance status, aggressive treatment, and potentially better tolerance leading to more exposure to antimyeloma therapy. The impact of EMD and ISS have been well described in MM, and the presence of EMD in this population remains an important prognostic factor. Lastly, although we used an arbitrary cut-off of 45 years old, we acknowledge this population may well represent biological spectrum of disease subtypes with potential differences in disease kinetics.
Disclosures
Monge:Bristol Myers Squibb: Consultancy. Niesvizky:Takeda: Consultancy, Research Funding; Karyopharm: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; GlaxoSmithKline: Consultancy, Research Funding; Janssen: Consultancy, Research Funding. Bustoros:Bristol Myers Squibb: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Meniarini: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal